Cancer-focused biotechnology company Kazia Therapeutics will present recent positive interim efficacy data on its lead program, paxalisib, at Biotech Showcase in San Francisco.
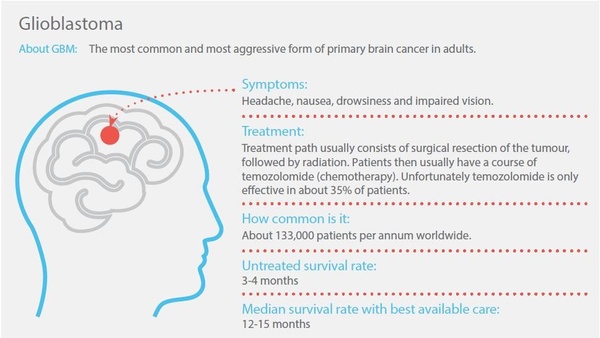
Paxalisib (formerly known as GDC-0084) is a small molecule inhibitor of the PI3K pathway, which is being developed to treat glioblastoma - the most common and most aggressive form of primary brain cancer in adults.
Glioblastoma is a devastating cancer, which has a survival rate of 12-15 months with best available treatments. It has no clear cause or strong risk factors and the indicative market opportunity for the disease is $US1.5 billion.
The only FDA-approved drug for glioblastoma, temozolomide, remains ineffective in around 65% of cases. Paxalisib is being developed for these patients for whom there is no effective pharmacological treatment currently available.
At Biotech Showcase, Kazia CEO Dr James Garner will speak about the positive data from the company’s ongoing phase II study of paxalisib in glioblastoma. The data, which was presented at the prestigious annual meeting of the Society for Neuro-Oncology in November 2019, showed a progression free survival in the eight patients treated of around 8.4 months, which compares very favourably to benchmarks for glioblastoma.
In addition, Dr Garner will speak about paxalisib being selected to join GBM AGILE, an international, academic-led, multi-drug adaptive phase II / III study in glioblastoma that will be used to seek registration for the drug.
“As well as the commencement of GBM AGILE, 2020 will be a milestone year for Kazia, with a number of value-driving data read-outs expected during the early part of the year, both from our own ongoing phase II study in glioblastoma and from our other studies in different forms of brain cancer,” Dr Garner said.
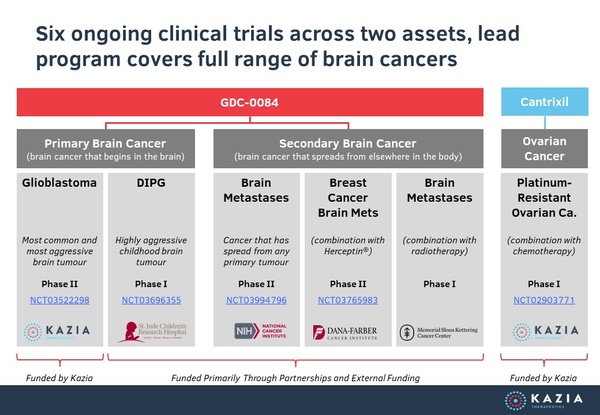
In addition to its phase II study, Kazia has four other clinical trials of paxalisib currently underway at leading US hospitals, and primarily funded by external parties, providing multiple shots on goal.
Dr Garner will present to investors on Monday 13 January at 2.30pm in the Franciscan A Ballroom at the Hilton Union Square Hotel.
The company presentation will be made available via the Kazia website and, subsequent to the meeting, through the Biotech Showcase website.
About Kazia Therapeutics Limited
Kazia Therapeutics Limited (ASX: KZA, NASDAQ: KZIA) is an innovative oncology-focused biotechnology company, based in Sydney, Australia. Our pipeline includes two clinical-stage drug development candidates, and we are working to develop therapies across a range of oncology indications.
Our lead program is paxalisib (GDC-0084), a small molecule inhibitor of the PI3K / AKT / mTOR pathway, which is being developed to treat glioblastoma, the most common and most aggressive form of primary brain cancer in adults. Licensed from Genentech in late 2016, paxalisib entered a phase II clinical trial in 2018. Interim data was reported in November 2019, and further data is expected in 1H 2020. Paxalisib was granted orphan designation for glioblastoma by the US FDA in February 2018.
TRX-E-002-1 (Cantrixil), is a third generation benzopyran molecule with activity against cancer stem cells and is being developed to treat ovarian cancer. TRX-E-002-1 is currently undergoing a phase I clinical trial in Australia and the United States. Interim data was presented at the ESMO Congress in September 2019, and the study remains ongoing. Cantrixil was granted orphan designation for ovarian cancer by the US FDA in April 2015.
For additional information, please visit: https://www.kaziatherapeutics.com/